PRESCRIPTION MEDICATION
Don’t let your hair loss define you.
Prescription hair loss medication delivered directly to your door.
GET STARTED

Topical Finasteride 0.3%
Topical finasteride is available off label to help prevent further hair loss.
Details ↓
It is a powerful 5-alpha reductase inhibitor, meaning it blocks the conversion of testosterone to DHT (dihydrotestosterone). High levels of the hormone DHT are a major contributing factor in the progression of androgenic alopecia. DHT is many times more potent than testosterone and binds to receptors in the DHT-sensitive zones of the scalp, promoting hair miniaturization and eventual hair loss. Finasteride (oral and topical) can significantly reduce the amount of DHT, thereby reducing future hair loss and protecting the hair you have. Decreasing the amount of DHT has been proven to help increase hair regrowth and slow hair loss on the scalp. Hair growth on other parts of the body is not affected by finasteride.
Instructions ↓
Apply up to 1 ml to the scalp daily or as prescribed. Apply only to areas affected by hair loss. Wash hands thoroughly after use. Practice chemical safety.
Warnings ↓
- Allergic reactions to any new medications may occur.
- Not FDA approved in topical form for hair loss.
- Potential side effects include: light-headedness, headaches, redness at the application site, scalp itching and irritation.
- Though less likely with topical preparation compared to oral form, potential side effects can include: decrease in sex drive, trouble getting or keeping an erection, decrease in the amount of semen, breast tenderness, testicular pain, depression.
- A very rare but serious potential complication includes increased levels of alanine transaminase which can compromise the liver and lead to frequent daytime urination and testicular pain.
- May be associated with an increased risk of high-grade prostate cancer.
- Finasteride is a teratogen and therefore should not be used by women who are or may be pregnant or those who may become pregnant, and therefore is typically not prescribed to pre-menopausal women. It also should not be used while breastfeeding.
- Finasteride can affect a blood test called PSA (Prostate-Specific Antigen) for the screening of prostate cancer.
Important safety information ↓
Topical finasteride is designed specifically to treat male pattern hair loss (androgenetic alopecia) in men. It’s effectiveness in bitemporal recession has not been proven, and it is not intended for use in women. This solution is to be applied topically to affected area only. The suggested usage is 1 ml applied to treatment area once daily. Generally, it takes at least three months of consistent use to see results, and ongoing use is necessary to maintain benefits. It is important to note that the topical application of finasteride is not as well studied as oral finasteride, and it is not currently FDA-approved. Topical finasteride has been shown to reduce both scalp and plasma DHT levels, and twice daily application of 0.25% finasteride solution has been shown to provide similar inhibition of plasma DHT as once-daily finasteride 1 mg oral tablet.1 Topical finasteride reduces the potential for systemic side effects, including the risk of sexual dysfunction. Periodic re-evaluation is advised. If treatment is discontinued, any positive effects will likely reverse within a year.
SIDE EFFECTS
As with any medication, some users may experience side effects. Topical application of finasteride decreases the chances of systemic effects compared to oral finasteride. Potential side effects include:
1. Side effects localized to the application site, including: scalp itchiness (pruritus), burning sensation, irritation, contact dermatitis, and erythema.
2. Decreased libido: Some users may experience a reduction in sexual desire.
3. Erectile dysfunction: Difficulty achieving or maintaining an erection may occur.
4. Ejaculation disorders: This may include decreased ejaculate volume or difficulty with ejaculation.
5. Testicular pain: Some men may experience pain or discomfort in the testicles.
6. Breast tenderness or enlargement: Changes in breast tissue, such as tenderness or swelling, may occur in some men.
7. Dizziness: Light-headedness or dizziness may occur, especially when standing up from a sitting or lying position.
8. Headaches: Some users may experience mild to moderate headaches.
9. Allergic reactions: These may include skin rash, itching, or hives, although these are less common.
10. Fatigue: Some users may experience increased tiredness or weakness.
11. Mood changes: This includes depression, anxiety, or other changes in mood, although these side effects are relatively rare.
It is important to note that not everyone will experience these side effects, and some people may experience no side effects at all. If you are concerned about any potential side effects, it is best to speak with your healthcare provider before starting or continuing the medication.
WARNINGS
1. Women's Exposure - Male Fetus Risk
Finasteride is not for women. Pregnant or potentially pregnant women should avoid handling the solution due to the risk of absorption and potential harm to a male fetus. Practice chemical safety when handling this prescription medication.
2. PSA Effects
In clinical studies with men aged 18 to 41, oral finasteride tablets reduced serum PSA levels. In older men with BPH, PSA levels decreased by about 50%. Finasteride may also lower PSA levels in prostate cancer patients. Consider these findings when interpreting PSA results for men on finasteride. Non-compliance may affect test results.
3. High-Grade Prostate Cancer Risk with 5-Reductase Inhibitors
Men aged 55+ in the 7-year PCPT with normal digital rectal exams and baseline PSA ≤3 ng/mL had an increased risk of high-grade prostate cancer when taking oral finasteride 5 mg/day. Similar results were found with dutasteride. The impact of 5α-reductase inhibitors on prostate volume or study-related factors is unclear.
4. Pediatric Patients
Finasteride is not for use in pediatric patients.
If you are experiencing a medical emergency, please call 911 or seek immediate medical attention.
You are encouraged to report negative side effects of prescription products to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
This information is not comprehensive. Please visit dailymed.nlm.nih.gov for more information.
REFERENCE USED: 1https://pubmed.ncbi.nlm.nih.gov/35238144/#:~:text=The%20side%20effects%20are%20localized,%2C%20contact%20 dermatitis%2C%20and%20 erythema.

Oral Finasteride 1mg
Finasteride is one of the most powerful medications available to prevent progressive male pattern hair loss. It was first approved for medical use in 1992 to treat benign prostatic hyperplasia.
Details ↓
Oral finasteride is FDA approved in the oral form for preventing hair loss. It is a powerful5-alpha reductase inhibitor, meaning it blocks the conversion of testosterone to DHT (dihydrotestosterone). High levels of the hormone DHT are a major contributing factor in the progression of androgenic alopecia. DHT is many times more potent than testosterone and binds to receptors in the DHT-sensitive zones of the scalp, promoting hair miniaturization and eventual hair loss. Finasteride (oral and topical) can significantly reduce the amount of DHT, thereby reducing future hair loss and protecting the hair you have. Decreasing the amount of DHT has been proven to help increase hair regrowth and slow hair loss on the scalp. Hair growth on other parts of the body is not affected by finasteride.
Instructions ↓
Take one tablet daily as prescribed.
Warnings ↓
- Allergic reactions to any new medications may occur.
- Potential side effects include: decrease in sex drive, trouble getting or keeping an erection, decrease in the amount of semen, breast tenderness, testicular pain, depression.
- A very rare but serious potential complication includes increased levels of alanine transaminase which can compromise the liver and lead to frequent daytime urination and testicular pain.
- May be associated with an increased risk of high-grade prostate cancer.
- Finasteride is a teratogen and therefore should not be used by women who are or may be pregnant or those who may become pregnant, and therefore is typically not prescribed to pre-menopausal women. It also should not be used while breastfeeding.
- Finasteride can affect a blood test called PSA (Prostate-Specific Antigen) for the screening of prostate cancer.
Important safety information ↓
Finasteride tablets are designed specifically to treat male pattern hair loss (androgenetic alopecia) in men. Their effectiveness in bitemporal recession has not been proven, and they are not intended for use in women. These tablets can be taken with or without food. The suggested dosage is one 1 mg tablet daily. Generally, it takes at least three months of consistent use to see results, and ongoing use is necessary to maintain benefits. Periodic re-evaluation is advised. If treatment is discontinued, any positive effects will likely reverse within a year.
SIDE EFFECTS
As with any medication, some users may experience side effects. The most common side effects of finasteride include:
1. Decreased libido: Some users may experience a reduction in sexual desire.
2. Erectile dysfunction: Difficulty achieving or maintaining an erection may occur.
3. Ejaculation disorders: This may include decreased ejaculate volume or difficulty with ejaculation.
4. Testicular pain: Some men may experience pain or discomfort in the testicles.
5. Breast tenderness or enlargement: Changes in breast tissue, such as tenderness or swelling, may occur in some men.
6. Dizziness: Lightheadedness or dizziness may occur, especially when standing up from a sitting or lying position.
7. Headaches: Some users may experience mild to moderate headaches.
8. Allergic reactions: These may include skin rash, itching, or hives, although these are less common.
9. Fatigue: Some users may experience increased tiredness or weakness.
10. Mood changes: This includes depression, anxiety, or other changes in mood, although these side effects are relatively rare.
It's important to note that not everyone will experience these side effects, and some people may experience no side effects at all. If you are concerned about any potential side effects, it's best to speak with your healthcare provider before starting or continuing the medication.
WARNINGS
1. Women's Exposure - Male Fetus Risk
Finasteride tablets are not for women. Pregnant or potentially pregnant women should avoid handling crushed or broken tablets due to the risk of absorption and potential harm to a male fetus. The tablets are coated to prevent contact with the active ingredient during normal handling.
2. PSA Effects
In clinical studies with men aged 18 to 41, finasteride tablets reduced serum PSA levels. In older men with BPH, PSA levels decreased by about 50%. Finasteride may also lower PSA levels in prostate cancer patients. Consider these findings when interpreting PSA results for men on finasteride. Non-compliance may affect test results.
3. High-Grade Prostate Cancer Risk with 5-Reductase Inhibitors
Men aged 55+ in the 7-year PCPT with normal digital rectal exams and baseline PSA ≤3 ng/mL had an increased risk of high-grade prostate cancer when taking finasteride 5 mg/day. Similar results were found with dutasteride. The impact of 5α-reductase inhibitors on prostate volume or study-related factors is unclear.
4. Pediatric Patients
Finasteride tablets are not for use in pediatric patients.
If you are experiencing a medical emergency, please call 911 or seek immediate medical attention.
You are encouraged to report negative side effects of prescription products to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
This information is not comprehensive. Please visit dailymed.nlm.nih.gov for more information.

Oral Minoxidil 2.5mg
Oral minoxidil is available off label to help boost hair growth
Details ↓
Oral minoxidil works by prolonging the anagen growth phase of the hair cycle and stimulating follicular proliferation – it encourages small, underdeveloped vellus hairs to differentiate into mature hairs. First developed in the 1950s to treat ulcers (and later blood pressure), minoxidil at low doses has been shown to increase hair growth for both men and women. Oral minoxidil may be more effective than topical minoxidil and once a day pill consumption carries a higher compliance rate compared with a twice a day topical application. Oral minoxidil does not affect hair texture or leave a residue on the scalp as minoxidil solutions and foam sometimes can.
Instructions ↓
- Take one tablet daily as prescribed.
Warnings ↓
- Allergic reactions to any new medications may occur.
- Not FDA approved in oral form for hair restoration.
- Potential side effects include: Hypertrichosis (including increased facial hair), leg swelling, effusions, low blood pressure, headaches, dizziness.
- Should be avoided in patients with an underlying cardiovascular condition.
- Initial hair loss or shedding is expected and typically resolves after one month.
- Results are not permanent, and medication use must be maintained to sustain effect.
Important safety information ↓
Oral minoxidil is a vasodilator medication primarily used to treat high blood pressure (hypertension) but has also been prescribed off-label for hair loss. Minoxidil for hair loss is prescribed at low doses to mitigate some of the risks.
SIDE EFFECTS
As with any medication, some users may experience side effects. The most common side effects of oral minoxidil include:
1. Unwanted hair growth: Some users may experience hair growth in areas other than the scalp, such as the face or other parts of the body.
2. Dizziness: Lightheadedness or dizziness may occur, especially when standing up from a sitting or lying position.
3. Fatigue: Some users may experience increased tiredness or weakness.
4. Headache: Some users may experience mild to moderate headaches.
5. Nausea or vomiting: Oral minoxidil may cause gastrointestinal upset in some users.
6. Fluid retention: This can lead to swelling (edema) in the extremities, such as the hands, feet, or ankles.
7. Heart palpitations: Some users may experience an increased heart rate or a sensation of a racing or pounding heartbeat.
8. Rapid weight gain: Sudden weight gain due to fluid retention may occur in some users.
9. Shortness of breath: Difficulty breathing, especially when lying down, may occur in some users.
10. Chest pain: Some users may experience chest pain or discomfort, although this is less common.
It's important to note that not everyone will experience these side effects, and some people may experience no side effects at all. If you are concerned about any potential side effects, it's best to speak with your healthcare provider before starting or continuing the medication.
WARNINGS
1. Salt and Water Retention: Minoxidil tablets must typically be used alongside a strong diuretic to prevent fluid retention and possible congestive heart failure. Monitoring body weight is essential. Without a diuretic, salt and water retention may occur, leading to edema. Diuretics, combined with salt restriction, usually minimize fluid retention. Refractory fluid retention might require discontinuation of minoxidil or temporary cessation followed by resuming treatment with aggressive diuretic therapy.
2. Preventing Tachycardia: Minoxidil increases heart rate and may worsen or induce angina due to increased oxygen demands. This can be prevented by administering a beta-adrenergic blocker or other sympathetic nervous system suppressant. Continuous effectiveness of the suppressant should be ensured.
3. Pericarditis, Pericardial Effusion, and Tamponade: Minoxidil has been associated with pericarditis, pericardial effusion, and tamponade in about 3% of treated patients, especially those with impaired renal function. Patients should be closely monitored for pericardial disorders, and appropriate interventions should be applied if necessary. If effusion persists, consider discontinuing minoxidil.
4. Interaction with Guanethidine: Minoxidil may cause profound orthostatic effects in patients receiving guanethidine. If possible, guanethidine should be stopped before starting minoxidil. If not, initiate minoxidil therapy in a hospital setting.
5. Rapid Control of Blood Pressure Risks: In patients with severe hypertension, rapid blood pressure control can cause syncope, cerebrovascular accidents, myocardial infarction, and sensory organ ischemia. Minoxidil initiation in malignant hypertension patients should occur in a hospital setting to monitor blood pressure changes.
6. In animal studies, minoxidil was found to induce various types of myocardial lesions and other negative cardiac consequences (see CARDIAC LESIONS IN ANIMALS).
If you are experiencing a medical emergency, please call 911 or seek immediate medical attention.
You are encouraged to report negative side effects of prescription products to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
This information is not comprehensive. Please visit dailymed.nlm.nih.gov for more information.

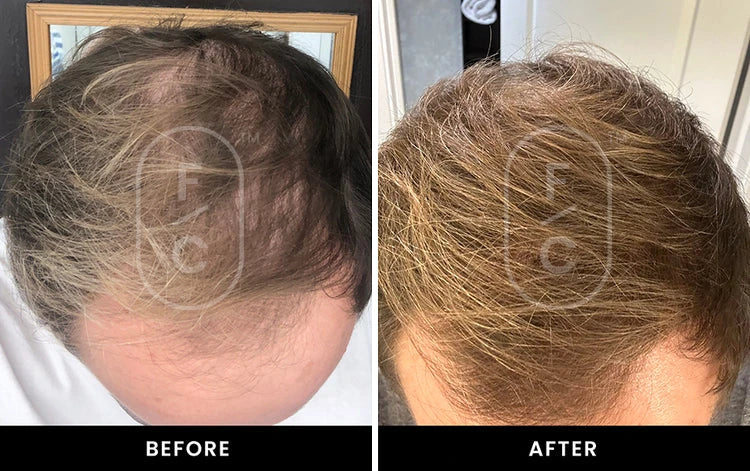
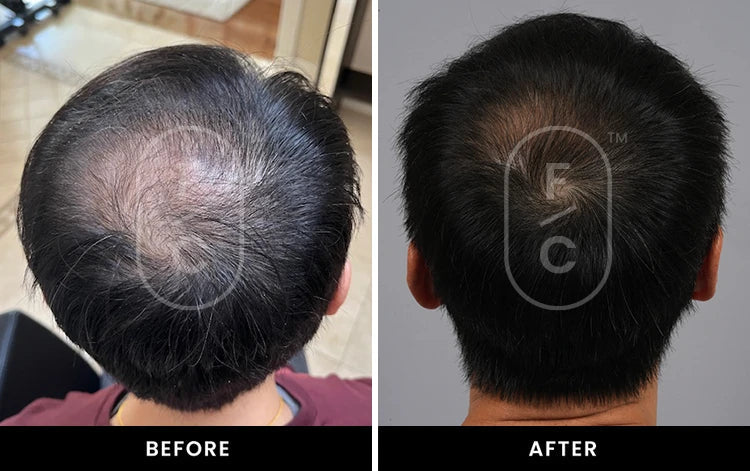
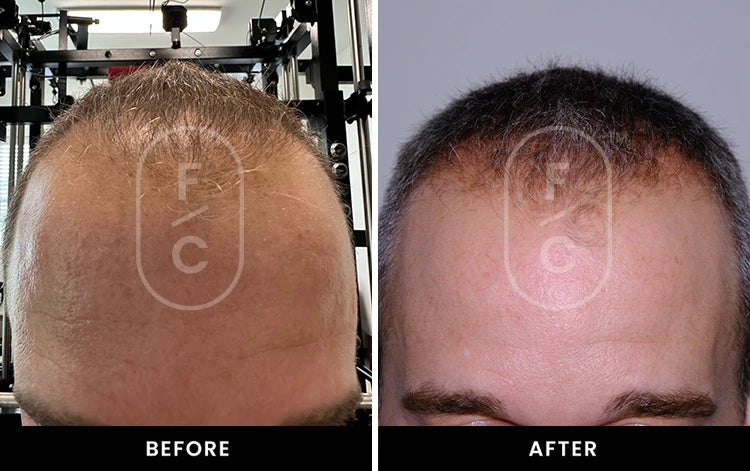
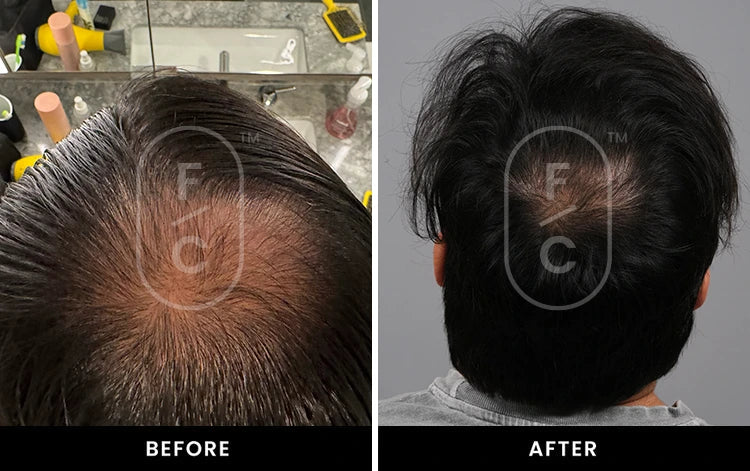
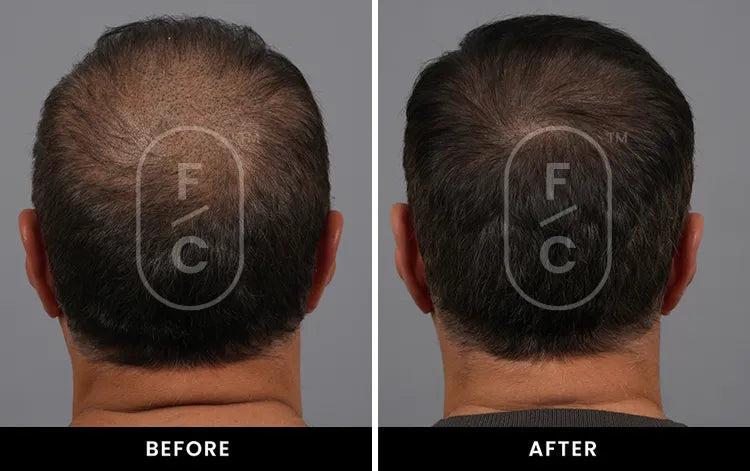
Real Results from Real Customers
Why Feel Confident?
Feel Confident
Round out your confident haircare routine